COMPARE AND CONTRAST THE ELEMENTS
This is the elements in the periodic
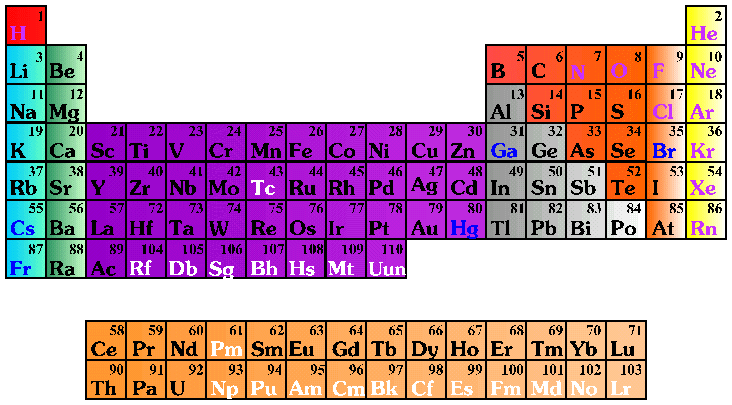 All known substances can be classified as solids, liquids, gases, or plasma. In addition, a fifth state of matter, the Bose-Einstein condensate has been discovered recently. However, it is not stable at normal earth conditions. Likewise, although plasma is the most abundant state of matter in the Universe, it is not common on the Earth under normal conditions, except for lightning. Most matter that students are familiar with will therefore be in a solid, liquid, or gaseous state.
All known substances can be classified as solids, liquids, gases, or plasma. In addition, a fifth state of matter, the Bose-Einstein condensate has been discovered recently. However, it is not stable at normal earth conditions. Likewise, although plasma is the most abundant state of matter in the Universe, it is not common on the Earth under normal conditions, except for lightning. Most matter that students are familiar with will therefore be in a solid, liquid, or gaseous state.
An element is a pure substance that cannot be decomposed into simpler substances by normal chemical means. There are 109 different elements. Ninety of these are naturally occurring; the rest have been created in laboratories. Elements 110 and 118 are still being researched on. There will be more elements as technology can identify them. A symbol is used to represent the full name of an element. For example, H represents hydrogen; O represents oxygen, and Al represents aluminum. Sometimes the Latin name for an element is used as the basis for its symbol, for instance K represents potassium (kalium in Latin).
Three subatomic particles compose elements: protons, neutrons, and electrons. Protons, which have an electrical charge of +1, and neutrons, which have a neutral charge, make up the nucleus of an element. This nucleus is surrounded by a "cloud" of electrons, each of which as a charge of -1. The electrons spin around the nucleus in what are called orbits or shells. Each of the orbits can contain a set number of electrons. For instance, the first orbital from the nucleus has 2 electrons, the second has 8, the third has 8, the 4th has 16 and the fifth has 32, and so on. Each shell may not be full, depending on the number of electrons in the element, and the inner shells fill before the outer shells fill. Sodium, for example, has 11 electrons, which are located in the first, second, and third shells (2+8+1.)
An element has a uniform composition. Different elements may join together; these combinations are called compounds. A compound can be separated into its component elements by chemical means. For example, common table salt is a compound made of two elements: sodium and chlorine. Table salt can be broken down into sodium and chlorine by mixing it with water. However, sodium and chlorine cannot be easily broken down into any simpler forms.
Definitions
Compare and contrast essays
are multi-paragraph compositions that explain ways in which two (or,
very occasionally, more) subjects are similar or different. In these papers, compare
means describing similarities between the subjects. Comparison in writing discusses elements that are similar, while contrast in writing discusses elements that are different. A compare-and-contrast essay, then, analyzes two subjects by comparing them, contrasting them, or both.
The key to a
good compare-and-contrast essay is to choose two or more subjects that
connect in a meaningful way. The purpose of conducting the comparison or
contrast is not to state the obvious but rather to illuminate subtle
differences or unexpected similarities. For example, if you wanted to
focus on contrasting two subjects you would not pick apples and oranges;
rather, you might choose to compare and contrast two types of oranges
or two types of apples to highlight subtle differences. For example, Red
Delicious apples are sweet, while Granny Smiths are tart and acidic.
Drawing distinctions between elements in a similar category will
increase the audience’s understanding of that category, which is the
purpose of the compare-and-contrast essay.
Similarly,
to focus on comparison, choose two subjects that seem at first to be
unrelated. For a comparison essay, you likely would not choose two
apples or two oranges because they share so many of the same properties
already. Rather, you might try to compare how apples and oranges are
quite similar. The more divergent the two subjects initially seem, the
more interesting a comparison essay will be.
VENN DIAGRAM
Useful when comparing and contrasting only two things
Useful when comparing and contrasting only two things
Writing at Work
Comparing and
contrasting is also an evaluative tool. In order to make accurate
evaluations about a given topic, you must first know the critical points
of similarity and difference. Comparing and contrasting is a primary
tool for many workplace assessments. You have likely compared and
contrasted yourself to other colleagues. Employee advancements, pay
raises, hiring, and firing are typically conducted using comparison and
contrast. Comparison and contrast could be used to evaluate companies,
departments, or individuals.
The Structure of a Comparison and Contrast
The
compare-and-contrast essay starts with a thesis that clearly states the
two subjects that are to be compared, contrasted, or both and the reason
for doing so. The thesis could lean more toward comparing, contrasting,
or both. Remember, the point of comparing and contrasting is to provide
useful knowledge to the reader. Take the following thesis as an example
that leans more toward contrasting.
-
There are two main organizing strategies for compare-and-contrast essays.
- Organize by the subjects themselves, one then the other.
- Organize by individual points, in which you discuss each subject in relation to each point.
- Use phrases of comparison or phrases of contrast to signal to readers how exactly the two subjects are being analyzed.
- Phrases of Comparison and Contrast
Comparison Contrast one similarity one difference another similarity another difference both conversely like in contrast likewise unlike similarly while in a similar fashion whereas Writing a Comparison and Contrast Essay
First choose whether you want to compare seemingly disparate subjects, contras seemingly similar subjects, or compare and contrast subjects. Once you have decided on a topic, introduce it with an engaging opening paragraph. Your thesis should come at the end of the introduction, and it should establish the subjects you will compare, contrast, or both as well as state what can be learned from doing so.The body of the essay can be organized in one of two ways: by subject or by individual points. The organizing strategy that you choose will depend on, as always, your audience and your purpose. You may also consider your particular approach to the subjects as well as the nature of the subjects themselves; some subjects might better lend themselves to one structure or the other. Make sure to use comparison and contrast phrases to cue the reader to the ways in which you are analyzing the relationship between the subjects.After you finish analyzing the subjects, write a conclusion that summarizes the main points of the essay and reinforces your thesis.
Source :
https://www.msnucleus.org/membership/html/k-6/rc/chemistry/3/rcc3_1a.html
https://saylordotorg.github.io/text_writing-for-success/s14-07-comparison-and-contrast.html
http://study.com/academy/lesson/compare-contrast-essay-definition-topics-examples.html









How the elements grouped on table periodic?
BalasHapusokay hanna, i will to try to be answer The periodic table arranges all of the known elements in order of increasing atomic number. Order generally coincides with increasing atomic mass. The different rows of elements are called periods. The period number of an element signifies the highest energy level an electron in that element occupies (in the unexcited state). The number of electrons in a period increases as one moves down the periodic table; therefore, as the energy level of the atom increases, the number of energy sub-levels per energy level increases.
HapusElements that lie in the same column on the periodic table (called a "group") have identical valance electron configurations and consequently behave in a similar fashion chemically. For instance, all the group 18 elements are inert, or noble gases.
Element groups are either nonmetals or various subsets of metals, but there is no distinct line between the two types of elements. Metal elements are usually good conductors of both electricity and heat. Subsets are based on similar characteristics and chemical properties. Our version of the periodic table uses the most commonly accepted demarcations between the elements.
Alkali metals: The alkali metals make up Group 1 of the table, and comprise lithium (Li) through francium (Fr). These elements have very similar behavior and characteristics. Hydrogen is Group 1, but it exhibits few characteristics of a metal and is often categorized with the nonmetals.
Alkaline earth metals: The alkaline earth metals make up Group 2 of the periodic table, from beryllium (Be) through radium (Ra). The alkaline earth metals have very high melting points and oxides that have basic alkaline solutions.
Lanthanides: The lanthanides comprise elements 57 — lanthanum (La), hence the name of the set — through 71, lutetium (Lu). They, along with the actinides, are often called "the f-elements" because they have valence electrons in the f shell.
Actinides: The actinides comprise elements 89, actinium (Ac), through 103, lawrencium (Lr). They, along with the lanthanides, are often called "the f-elements" because they have valence electrons in the f shell. Only thorium (Th) and uranium (U) occur naturally with significant abundance. They are all radioactive.
Transition metals: The transition elements are metals that have a partially filled d subshell and comprise Groups 3 through 12 and the lanthanides and actinides.
Post-transition metals: The post-transition elements are aluminum (Al), gallium (Ga), indium (In), thallium (Tl), Tin (Sn), lead (Pb) and bismuth (Bi). As the name implies, these elements have some of the characteristics of the transition metals, but they tend to be softer and conduct more poorly than the transition metals.
Metalloid: The metalloids are boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te) and polonium (Po). They sometimes behave as semiconductors (B, Si, Ge) rather than as conductors. Metalloids are also called "semi-metals" or "poor metals."
Nonmetals: The term "nonmetals" is used to classify hydrogen (H), carbon (C), nitrogen (N), phosphorus (P), oxygen (O), sulfur (S) and selenium (Se).
Halogens: The halogen elements are a subset of the nonmetals. They comprise Group 17 of the periodic table, from fluorine (F) through astatine (At). They are generally very chemically reactive and are present in the environment as compounds rather than as pure elements.
Noble gases: The inert, or noble, gases comprise Group 18. They are generally very stable chemically and exhibit similar properties of being colorless and odorless.
hi rini , after i read your article now i understand how to compare the element, and i want to remember the element of the periodic table , can you give me trick or tips to remember the element ? for group A or transision it depend on you thankss
BalasHapusOKAY, thanks eko, i have many trick to remenber the elements, how can? you just remember the formula of the blok, for example blok A group 1A the formula is S1 and the others, if blok B i just remember the formula for group 3B that is ns1(n-1)d1 and the others, so i hope you can practice the trick and tips about the elements
Hapuswhat is plasma ? can you explain more specific ?
BalasHapusokay vicky thanks for your comment i will to answer
HapusPlasma is considered the fourth state of matter. The three other states are solid, liquid, and gas. Plasma is a cloud of protons, neutrons and electrons where all the electrons have come loose from their respective molecules and atoms, giving the plasma the ability to act as a whole rather than as a bunch of atoms. A plasma is more like a gas than any of the other states of matter because the atoms are not in constant contact with each other, but it behaves differently from a gas. It has what scientists call collective behavior. This means that the plasma can flow like a liquid or it can contain areas that are like clumps of atoms sticking together.
Plasma is considered the fourth state of matter. The three other states are solid, liquid, and gas. Plasma is a cloud of protons, neutrons and electrons where all the electrons have come loose from their respective molecules and atoms, giving the plasma the ability to act as a whole rather than as a bunch of atoms. A plasma is more like a gas than any of the other states of matter because the atoms are not in constant contact with each other, but it behaves differently from a gas. It has what scientists call collective behavior. This means that the plasma can flow like a liquid or it can contain areas that are like clumps of atoms sticking together.
What are the elements on earth? And which elements are beneficial to our body?
BalasHapusThe Earth consists of a large collection of gases. Earth's atmospheric gas gases have many benefits for human life
Hapus1. Nitrogen
Is the largest gas element percentage in the atmosphere. Nitrogen gas is needed in plant growth. Nitrogen gas is often also used as the base material of the fertilizer industry. Nitrogen is a part of the living cell and the main part of all proteins, enzymes and metabolic processes that are included in the synthesis and transfer of energy.
Nitrogen is part of the chlorophyll, the green dye of the plant responsible for photosynthesis.Helping plants accelerate their growth, increase the production of seeds and fruit and improve the quality of leaves and roots.
The Haber-Bosch process is an industrial process for the manufacture of ammonia from nitrogen and hydrogen by combining them under high pressure in the presence of the iron catalyst.
2. Oxygen
Oxygen is an indispensable gas element for human respiration and other living things such as animals and plants. The oxygen composition in the atmosphere reaches 21%, oxygen is present in waters, especially shallow sea waters and inland to a certain height above sea level, the higher the area of a sea surface, the oxygen layer is thinner. Because there is oxygen we can breathe, light candles and others.
3. Argon
Argon symbol Ar, is the third largest gas element in Earth's atmosphere after the element of nitrogen and oxygen gas. The name of the Argon element, taken from Argos Greek which means inactive, because Argon is not easy to react with other elements. Argon is used in conjunction with Neon gas in the electrical industry to fill fluorescent lamps. Argon gas is blue. Fluorescent lamps filled with Argon Gas are more power-efficient than regular electric lights.
4.Carbon dioxide (CO2)
Carbon dioxide is a colorless, odorless gas and a light acid gas. Carbon dioxide is also called carbon-carbon gas, the molecule consists of 1 carbon atom and 2 oxygen atoms, symbolized CO2. Carbon dioxide is often called mixed air.
Some of the benefits of Carbon Dioxide Gas:
Carbon dioxide is used to produce Sodium Carbonate Na2CO3, sodium bicarbonate NaHC03 and other chemicals that can be utilized for human benefit.
Carbon dioxide gas is non combustible and can localize heat, that is why it is used as a filler for fire extinguishers.
Gas Carbon dioxide is needed in plant breathing and photosynthesis.
In addition to having harmful effects, CO2 also has benefits as for the benefits of CO2 sediri are as follows:
CO2 benefits can also be encountered in the process of making bread that serves as a bread developer with the help of yeast.
In the process of photosynthesis
Fire extinguishers
The carbon dioxide discharged in the fire through the fire hose will immediately blanket the fire, so that the fire will not be exposed to contact with oxygen so that the combustion will stop, because the burning stops, then the fire can be immediately extinguished.
5. Neon
Neon is a gas that is colorless, odorless and non reactive. Neon includes noble gases such as Helium, Argon, Krypton, Xenon and Radon.
Use of Neon Gas is as follows:
Utilized for the City neon lights at night
Ad purposes Can be used for high voltage indicators
Utilized in the medical world, for example to help see the x-rays
6. Helium
Helium comes from the Greek Helios, which means the Sun. Helium includes noble gases, lighter than air, so it is used for gas balloon and air balloon fillers. Helium is also used for fuel that can move a rocket engine.
Hydrogen is used in the chemical industry, to make ammonia (NH3), the usefulness of ammonia, among others, to make fertilizer.
Metallurgists use hydrogen to separate pure metals with oxides, for example, to produce copper sheets.
What compare between Radon and Uranium?
BalasHapus